KCNK9 (TASK3, K2P9.1) belongs to the TASK (TWIK-related acid-sensitive K+ channel) subfamily of two-pore domain potassium (K2P) channels. It is highly expressed in the central nervous system and has been shown to play an important role in the migration of cortical pyramidal neurons during development. The KCNK9 gene is unique among the K2P family as it is genetically imprinted, with a monoallelic, parental-specific expression pattern. Only 1% of genes in the mammalian genome are known to be imprinted. Despite this low occurrence, it is now becoming evident that hereditary or de novo mutations of these imprinted genes, will lead to dysfunction and often result in human diseases which affect development and behavior. A single missense mutation of a glycine (G) at position 236, to an arginine (R) in KCNK9 has been linked to the rare Mendelian disease, KCNK9 imprinting syndrome, for which there is currently no therapeutic treatment. The original description of this disease-causing mutation in an Arab-Israeli family by Barel O et al. (2008), showed that the mutated channel was non-functional and could act as a dominant-negative to produce a four-fold reduction in current through a related channel, KCNK3 (TASK1, K2P3.1). A later study showed that transient knockdown of KCNK9 in the cerebral cortex during development resulted in severe impairment of migrating cortical pyramidal neurons (Bando Y et al. 2014). The cerebellum has traditionally been known to play a pivotal role in motor function. However, there is increasing evidence that the cerebellum is also involved in cognition and emotion and more recent evidence suggests the involvement of the cerebellum in the pathophysiology of psychiatric disorders, such as autism and schizophrenia. In contrast to Barel O et al. (2008), Veale EL et al. (2014) showed, using whole-cell patch clamp electrophysiology, that the mutated channels were not completely non-functional but that a small, albeit heavily reduced, current passed through the G236R mutant channel. One of the striking characteristics identified was, in contrast to WT KCNK9 channels, that the current became inwardly rectifying, passing more inward current than outward. Current through the G236R mutant channels could be partially recovered by a family of non-steroidal anti-inflammatory drugs (NSAIDs), flufenamic acid (FFA) and to a lesser extent by mefenamic acid (MFA), drugs commonly used in the treatment of pain (see Veale EL et al. 2014 and figure 1, Veale & Mathie, unpublished data). These drugs were found to have little or no effect on the wild type (WT) TASK3 channels.
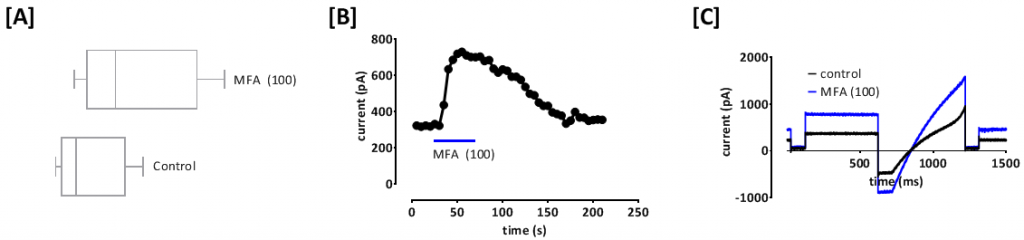
Figure 1: Mefenamic acid (MFA) is able to partially recover current through poorly functioning human TASK3_G236R mutant channels. [A] Box and whisker plot of current through G236R channels in the presence and absence of MFA (100 µM). [B] Time-course plot showing the acute application of 100 µM MFA (blue line) on G236R current. [C] Exemplar data trace from G236R mutant channels in the absence (black line) and presence of 100 µM MFA (blue line).
As a result of this work, MFA has been used clinically in the treatment of a small number of patients with the G236R mutation, with noted moderate therapeutic benefit and no observed adverse events (Graham JM Jr et al. 2016). The development of novel, selective pharmacological agonists that enhance current through poorly functioning ion channels, could potentially provide therapeutic benefit for patients. At present, there are few known TASK channel activators, however, recently, two new activators of KCNK9 channels have been identified. The first of these was the antifungal agent, terbinafine, which was shown to activate TASK3 channels in a thallium flux assay. This was replicated in whole cell patch clamp electrophysiology where the compound was shown to be a stronger activator of G236R mutated TASK3 channels than WT TASK3 channels (Wright PD et al. 2017).
The second compound was a novel small molecule, NPBA (N-(2-159 ((4-nitro-2-(trifluoromethyl)phenyl)amino)ethyl)benzamide) which has been shown to be a selective WT TASK3 channel activator with little effect on TASK1 channels. Amino acid residues on TASK3 channels that may be involved in the mechanistic effect of this compound have been identified (Tian F et al. 2019).
Whole exome sequencing has identified a series of patients with novel variant mutations located at different regions of the TASK3 channel but with symptoms overlapping with KCNK9 imprinting syndrome patients who have the G236R mutation. Work is currently been undertaken to characterize these individual mutations, using whole-cell patch clamp electrophysiology and in-silico modelling. A publication of these experimental and clinical findings is expected to be released in late 2019, whilst clinical findings of one of these novel variants, A237D have already been reported (Šedivá M et al. 2019).
With the advent of routine whole exome sequencing and better clinical reporting, including web pages such as this, it is likely that the number of cases reported will grow considerably. For other rare Mendelian diseases, specific DNA methylation episignatures have been identified which are enabling diagnosis, screening and genetic variation classifications of other syndromes and may be useful in KCNK9 imprinting syndrome (Bend EG et al. 2019). This condition certainly requires the development of reliable experimental models that can examine cortical neurons at different stages of development and the effect of novel small molecule activators of TASK3 channels with therapeutic potential in these models. Alongside this, a more in-depth study of the regulation of these channels, physiologically, is required.
Currently, a clinical registry is being assembled for affected individuals with de novo or maternally inherited KCNK9 variants and their families. The purpose of this study is to better understand the pathogenic genetic variation in KCNK9 and the spectrum of the clinical manifestations. In parallel, in silico molecular modelling and heterologous electrophysiology studies are being pursued for KCNK9 alterations to determine their effect on TASK3 channel function.
